In the world of chemistry, molecules are the building blocks of all matter. However, not all molecules are created equal. Some have formulas that are non-unique, meaning they can be represented by the same molecular formula but differ in structure or properties. This phenomenon is crucial for understanding chemical diversity and has significant implications in fields like medicine, materials science, and environmental studies.
This article explores the concept of molecules with non-unique formulas, focusing on isomers—compounds that share the same molecular formula but exhibit different structures and properties. We’ll delve into the science behind these fascinating substances, their classification, and why they matter in real-world applications.
What Are Molecules With Non-Unique Formulas?
A molecule is defined as a group of two or more atoms bonded together, representing the smallest unit of a chemical compound. While most compounds have unique molecular formulas, some molecules can have the same molecular formula but different arrangements of atoms, leading to distinct physical and chemical properties. These are known as isomers.
Isomerism is a fundamental concept in organic chemistry. It allows for a wide variety of compounds with identical compositions but differing behaviors. For example, glucose and fructose both have the molecular formula C₆H₁₂O₆, yet they have completely different structures and functions in the human body.
Types of Isomers
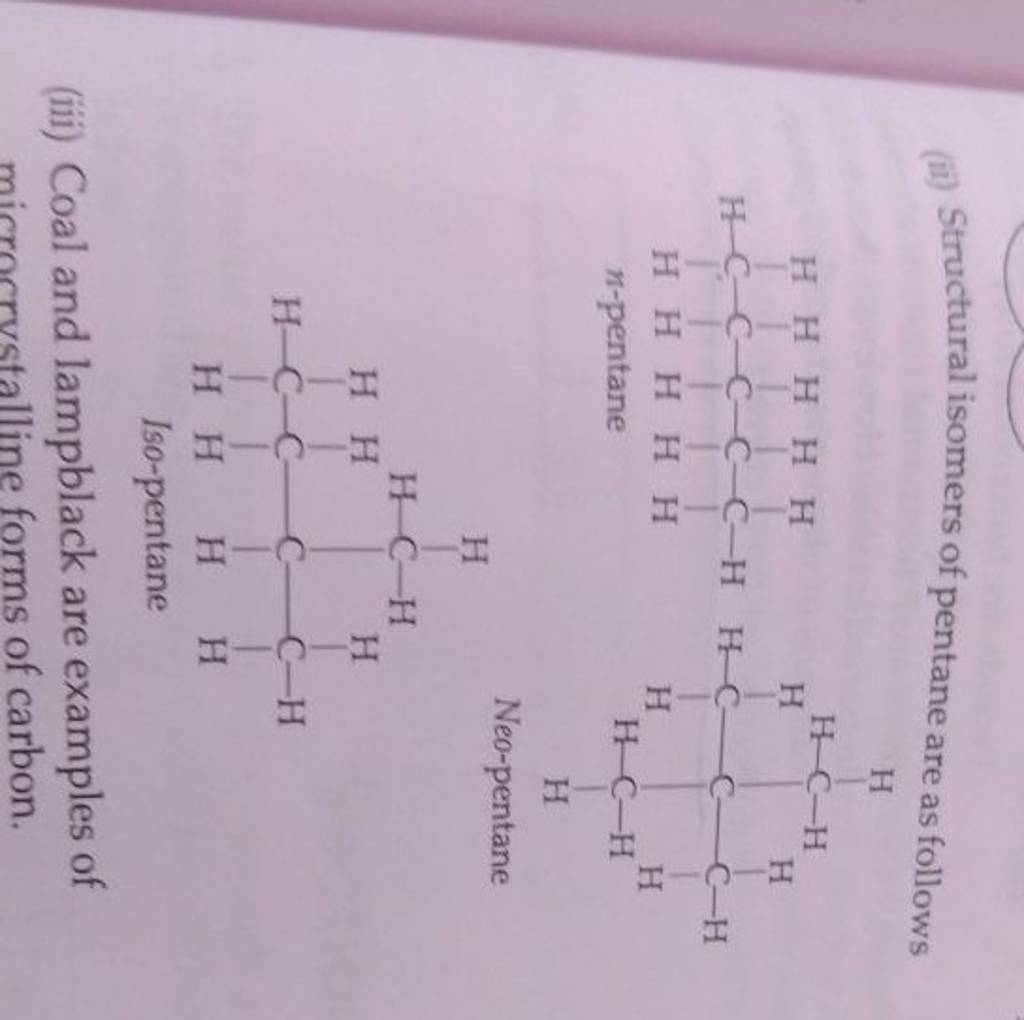
Isomers can be broadly categorized into two main types: structural isomers and stereoisomers.
1. Structural Isomers
Structural isomers differ in the connectivity of atoms within the molecule. This means the order in which atoms are linked can vary, resulting in different chemical properties.
Examples include:
- Chain isomerism: Differences in the carbon backbone. For instance, pentane (C₅H₁₂) can exist as n-pentane, iso-pentane, or neopentane.
- Position isomerism: The position of functional groups or substituents changes. For example, propanol can have the hydroxyl group at different positions along the carbon chain.
- Functional group isomerism: Different functional groups are present. For example, ethanol (an alcohol) and dimethyl ether (an ether) have the same molecular formula (C₂H₆O) but different structures and properties.
2. Stereoisomers

Stereoisomers have the same connectivity of atoms but differ in the spatial arrangement of atoms. This type of isomerism is especially important in biochemistry and pharmacology.
Examples include:
- Geometric isomers: Differ in the spatial orientation of atoms around a double bond. For example, cis- and trans-1,2-dichloroethene.
- Optical isomers: Mirror-image molecules that cannot be superimposed. These are often found in chiral molecules, such as amino acids and sugars.
Why Do Non-Unique Formulas Matter?

Molecules with non-unique formulas play a critical role in various scientific and industrial applications. Here’s why:
1. Drug Development
Many drugs are chiral, meaning they have optical isomers. One isomer may be effective while the other could be harmful or inactive. For example, thalidomide, a drug once used to treat morning sickness, had one isomer that was therapeutic and another that caused severe birth defects. Understanding isomerism is essential for safe and effective drug design.
2. Material Science

The properties of materials can change drastically based on isomerism. For example, polyethylene can exist in different forms (such as high-density and low-density polyethylene), each with unique mechanical and thermal properties due to variations in molecular structure.
3. Environmental Impact
Isomers can behave differently in the environment. For example, certain pesticides may have isomers that are more toxic to non-target species or persist longer in the ecosystem. Understanding this helps in developing safer and more sustainable chemicals.
Real-World Examples of Molecules With Non-Unique Formulas
Let’s look at a few common examples of molecules with non-unique formulas:
1. Glucose vs. Fructose
Both have the molecular formula C₆H₁₂O₆ but differ in the arrangement of hydroxyl (-OH) groups. Glucose is a primary energy source in the body, while fructose is commonly found in fruits and processed foods.
2. Acetylene and Benzene
These hydrocarbons share the same empirical formula (CH), but their molecular formulas are C₂H₂ and C₆H₆, respectively. Acetylene is used in welding, while benzene is a key component in plastics and solvents.
3. Lactic Acid Isomers
Lactic acid exists in two optical isomers: L-lactic acid (found in muscles during exercise) and D-lactic acid (used in food production). Their different properties make them suitable for different applications.
The Role of Molecular Formula in Chemistry
While the empirical formula gives the simplest whole-number ratio of elements in a compound, the molecular formula specifies the exact number of each atom. For molecules with non-unique formulas, the molecular formula is crucial because it provides a more accurate representation of the compound’s composition.
For example, acetylene (C₂H₂) and benzene (C₆H₆) have the same empirical formula (CH), but their molecular formulas reveal their true complexity and behavior.
Conclusion
Molecules with non-unique formulas, particularly isomers, highlight the complexity and diversity of chemical structures. Understanding these differences is essential in fields ranging from medicine to materials science. As research continues to advance, the study of isomerism will remain a cornerstone of modern chemistry, driving innovation and improving our ability to manipulate matter at the molecular level.
Whether you’re a student, a researcher, or simply curious about the science behind everyday substances, exploring the world of isomers offers fascinating insights into the hidden intricacies of chemistry.
Author Section
Author: Dr. Emily Carter
Title/Role: Senior Chemist and Science Writer
Credentials: Dr. Carter holds a Ph.D. in Organic Chemistry from Stanford University and has over 15 years of experience in chemical research and education. She specializes in molecular structure and isomerism, with numerous publications in peer-reviewed journals.
Profile Link: LinkedIn Profile
References
- National Institute of Standards and Technology (NIST)
- Chemistry LibreTexts – Isomerism
- ScienceDirect – Molecular Structure and Isomerism
Internal Links
Call to Action
Stay updated with the latest news and discoveries in chemistry and beyond. Explore today’s headlines and uncover the science behind the world we live in.











More Stories
What Is Yodo Para Tiroides and How Does It Affect Thyroid Health?
How to Claim Your Joy in League of Legends: A Step-by-Step Guide
What is WSET? A Comprehensive Guide to Wine Education